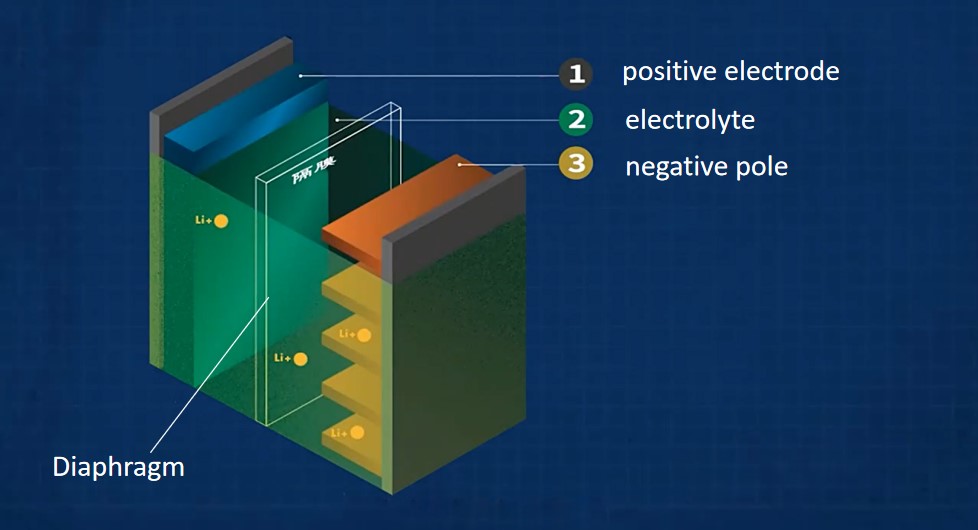
Ibhethri lensimbi-lomoya liwumsebenzi osebenzayo esebenzisa izinsimbi ezinamandla angasebenzi kahle e-electrode, njenge-magnesium, i-aluminium, i-zinc, i-mercury nensimbi, njenge-electrode eyinegethivu, nomoya-mpilo noma umoya-mpilo omsulwa emoyeni njenge-electrode ephozithivu.Ibhethri le-Zinc-air ibhethri elicwaningwe kakhulu nelisetshenziswa kakhulu ochungechungeni lwebhethri lomoya wensimbi.Eminyakeni engu-20 edlule, ososayensi benze ucwaningo oluningi ngebhethri lesibili le-zinc-air.I-Sanyo Corporation yase-Japan ikhiqize umthamo omkhulu webhethri lesibili le-zinc-air.Ibhethri le-zinc-air likagandaganda onamandla angu-125V namandla angu-560A · h lakhiwe ngokusebenzisa indlela yomoya kanye ne-electro-hydraulic force circulation.Kubikwa ukuthi isetshenziswe ezimotweni, futhi umthamo wayo wamanje wokukhipha ungafinyelela ku-80mA/cm2, kanti umkhawulo ungafinyelela ku-130mA/cm2.Ezinye izinkampani eFrance naseJapane zisebenzisa indlela yokujikeleza i-zinc slurry ukukhiqiza i-zinc-air yesibili yamanje, futhi ukubuyiswa kwezinto ezisebenzayo kwenziwa ngaphandle kwebhethri, ngamandla angempela aqondile angu-115W · h/kg.
Izinzuzo eziyinhloko zebhethri yensimbi yomoya:
1) Amandla athile aphezulu.Njengoba into esebenzayo esetshenziswa ku-electrode yomoya i-oxygen emoyeni, ayipheli.Ngokombono, umthamo we-electrode eqondile awupheli.Ngaphezu kwalokho, into esebenzayo ingaphandle kwebhethri, ngakho-ke amandla athiyelekile ebhethri yomoya makhulu kakhulu kunalawo e-electrode yensimbi evamile.Amandla athile etiyetha ebhethri lomoya lensimbi ngokuvamile angaphezulu kuka-1000W · h/kg, okungamandla asetshenziswa ngamakhemikhali anamandla.
(2) Intengo ishibhile.Ibhethri le-zinc-air alisebenzisi izinsimbi eziyigugu ezibizayo njengama-electrode, futhi izinto zebhethri ziyizinto ezivamile, ngakho intengo ishibhile.
(3) Ukusebenza okuzinzile.Ikakhulukazi, ibhethri le-zinc-air lingasebenza ngokuminyana okuphezulu kwamanje ngemuva kokusebenzisa i-porous zinc electrode kanye ne-alkaline electrolyte.Uma i-oxygen ehlanzekile isetshenziselwa ukushintsha umoya, ukusebenza kokukhipha kungathuthukiswa kakhulu.Ngokwezibalo zetiyori, ukuminyana kwamanje kungakhuphuka izikhathi ezingaba ngu-20.
Ibhethri lomoya wensimbi linobubi obulandelayo:
I-1), ibhethri alikwazi ukuvalwa, okulula ukubangela ukomiswa nokukhuphuka kwe-electrolyte, okuthinta umthamo nempilo yebhethri.Uma i-alkaline electrolyte isetshenziswa, kulula futhi ukubangela i-carbonation, ukwandisa ukumelana kwangaphakathi kwebhethri, futhi kuthinte ukukhishwa.
2), ukusebenza kwesitoreji esimanzi akulungile, ngenxa yokuthi ukusakazeka komoya ebhethrini kuya ku-electrode engalungile kuzosheshisa ukuzikhipha kwe-electrode eyinegethivu.
3), ukusetshenziswa kwe-zinc enezimbotshana njengoba i-electrode engalungile idinga i-mercury homogenization.I-Mercury ayigcini nje ngokulimaza impilo yabasebenzi kodwa futhi ingcolisa imvelo, futhi idinga ukushintshwa yi-non-mercury corrosion inhibitor.
Ibhethri lensimbi-lomoya liwumsebenzi osebenzayo esebenzisa izinsimbi ezinamandla angasebenzi kahle e-electrode, njenge-magnesium, i-aluminium, i-zinc, i-mercury nensimbi, njenge-electrode eyinegethivu, nomoya-mpilo noma umoya-mpilo omsulwa emoyeni njenge-electrode ephozithivu.Isixazululo samanzi se-alkaline electrolyte ngokuvamile sisetshenziswa njengesixazululo se-electrolyte sebhethri lomoya lensimbi.Uma i-lithium, i-sodium, i-calcium, njll. enamandla e-electrode angemuhle kakhulu isetshenziswa njenge-electrode engeyinhle, ngoba ingasabela emanzini, i-electrolyte ephilayo engenamanzi kuphela njenge-electrolyte eqinile engamelana ne-phenol noma i-electrolyte ye-inorganic njengesixazululo sikasawoti se-LiBF4 kusetshenziswe.
Ibhethri le-Magnesium-air
Noma yiliphi ipheya lensimbi elinamandla e-electrode elingalungile kanye ne-electrode yomoya ingakha ibhethri lomoya lensimbi elihambisanayo.Amandla e-electrode e-magnesium mancane kabi kanti i-electrochemical elingana nayo incane uma kuqhathaniswa.Ingasetshenziselwa ukubhanqa ne-electrode yomoya ukwakha ibhethri lomoya le-magnesium.I-electrochemical okulingana ne-magnesium ngu-0.454g/(A · h) Ф=- 2.69V. Amandla athiyori akhethekile webhethri lomoya we-magnesium ngu-3910W · h/kg, okuyizikhathi ezi-3 kunebhethri le-zinc-air kanye no-5~ Izikhathi ezingu-7 zalezo zebhethri ye-lithium.Isigxobo esingalungile sebhethri ye-magnesium-air yi-magnesium, isigxobo esihle siwumoya-mpilo emoyeni, i-electrolyte isisombululo se-KOH, kanye nesisombululo se-electrolyte esingathathi hlangothi singasetshenziswa.
Amandla ebhethri amakhulu, amandla ezindleko eziphansi kanye nokuphepha okuqinile kuyizinzuzo ezibalulekile zamabhethri e-magnesium ion.Isici se-divalent se-magnesium ion senza kube nokwenzeka ukuthwala nokugcina amashaji kagesi amaningi, nge-theoretical energy density izikhathi ezingu-1.5-2 zebhethri ye-lithium.Ngesikhathi esifanayo, i-magnesium kulula ukuyikhipha futhi isatshalaliswe kabanzi.I-China inenzuzo ephelele yokunikezwa kwezinsiza.Ngemuva kokwenza ibhethri le-magnesium, inzuzo yayo yezindleko ezingaba khona kanye nemfanelo yokuphepha kwezinsiza ziphakeme kunebhethri ye-lithium.Mayelana nokuphepha, i-magnesium dendrite ngeke ivele esigxotsheni esinegethivu sebhethri le-magnesium ion phakathi nomjikelezo wokushaja nokukhipha, ongagwema ukukhula kwe-lithium dendrite ebhethri ye-lithium ukubhoboza i-diaphragm futhi kubangele ibhethri ukuthi lihambe kancane, umlilo kanye ukuqhuma.Izinzuzo ezingenhla zenza ibhethri le-magnesium libe namathemba amahle okuthuthuka namandla.
Mayelana nokuthuthukiswa kwakamuva kwamabhethri e-magnesium, i-Qingdao Institute of Energy ye-Chinese Academy of Sciences yenze inqubekelaphambili enhle kumabhethri esibili e-magnesium.Njengamanje, iphule ibhodlela lobuchwepheshe enqubweni yokukhiqiza amabhethri esibili e-magnesium, futhi ithuthukise iseli elilodwa elinamandla amakhulu angu-560Wh/kg.Imoto kagesi enebhethri lomoya eliphelele le-magnesium elakhiwe eSouth Korea ingashayela ngempumelelo amakhilomitha angu-800, okuphindwe kane kunobubanzi obumaphakathi bezimoto zamanje ezinebhethri ye-lithium.Izikhungo eziningi zase-Japan, okuhlanganisa i-Kogawa Battery, i-Nikon, i-Nissan Automobile, i-Tohoku University yase-Japan, i-Rixiang City, i-Miyagi Prefecture, nezinye izikhungo zocwaningo lwemboni-inyuvesi kanye neminyango kahulumeni zikhuthaza ngenkuthalo ucwaningo lomthamo omkhulu webhethri yomoya ye-magnesium.U-Zhang Ye, iqembu locwaningo le-Modern Engineering College yaseNanjing University, kanye nabanye baklama ijeli ye-electrolyte enezingqimba ezimbili, eyaqaphela ukuvikelwa kwe-magnesium metal anode kanye nokulawulwa kwemikhiqizo ekhishwayo, futhi bathola ibhethri lomoya le-magnesium elinamandla amakhulu ( 2282 W h · kg-1, okusekelwe kwikhwalithi yawo wonke ama-electrode omoya nama-electrode e-magnesium negative), ephakeme kakhulu kunebhethri yomoya ye-magnesium enamasu okusebenzisa i-anode ne-anti-corrosion electrolyte ezincwadini zamanje.
Sekukonke, ibhethri le-magnesium lisesesigabeni sokuqala sokuhlola okwamanje, futhi kusenendlela ende okufanele yenziwe ngaphambi kokukhushulwa nokusetshenziswa kwezinga elikhulu.
Isikhathi sokuthumela: Feb-17-2023